copyright 2001, David M. Baker, Electron Microscope Lab, University of California, Berkeley
David M. Baker
MCB481C
Description and Interpretation of Stipule Development in Exbucklandia populnea (Hamamelidaceae)
Purpose
Obtaining
high-resolution images of the apical region of Exbucklandia populnea
to provide a context for interpreting histological sections being generated in a related project studying the
branching rhythm of the plant provided the impetus for the following work. The purpose of the work presented below was
to observe, describe, and interpret the development of the protective stipular
structure exhibited by individuals of Exbucklandia. Technical goals of the project included:
becoming competent in the preparation of specimens for viewing by scanning
electron microscopy; becoming competent in the operation of the scanning
electron microscope; and becoming proficient at recording the images obtained
by Polaroid film and digital image capture.
Introduction
Exbucklandia populnea (Hamamelidaceae),
is a tree that exhibits a flushing growth habit in which a few new leaves
expand at an elongating shoot tip simultaneously between intervals of
dormancy. Each shoot is not tipped by
an apical bud of scales or small expanding leaves, but by an asymmetrical green
rudder-shaped structure comprised of what has been interpreted as fused lateral
stipules of the subtending leaf3 (see figures 1 and 2). When dissected, this rudder structure splits
into two equal flaps along a median fissure line to reveal a number of
unexpanded shoots in a mat of trichomes. Observations and interpretations of the development of this protective
stipular structure are presented below. Commentary on the development of the leaf as a whole is also included. As alluded to above, the work
presented here is a part of a larger project of describing and morphologically
interpreting the development of the shoot apical region, here defined as the
protective stipular structure and the enclosed apical meristem with its
developing derivatives.
Materials and Methods
Six
shoot apices, consisting of a lamina, petiole, and protective stipulate
structure, containing developing shoots, were collected from the UC Berkeley
Botanical Garden and brought to the UCB Electron Microscope Laboratory. The lamina and petiole of the leaves were
removed and the stipulate structure was dissected to expose the developing
shoots within. The developing shoots
were fixed for two hours in 2% gluteraldehyde in 0.1M sodium cacodylate buffer,
pH 7.2. The samples were rinsed for
three fifteen-minute intervals with 0.1M sodium cacodylate buffer, pH 7.2. Two of the six samples were treated with 1%
osmium tetroxide in 0.1 M sodium cacodylate buffer, pH 7.2, for one hour
following fixation. Following this
treatment, the two osmium tetroxide treated samples were rinsed for three
five-minute intervals in cacodylate buffer, pH 7.2. All of the samples were dehydrated in a seven step dehydration
series, for 10 minutes per solution with ethanol concentrations of 35%, 50%,
70%, 80%, 95%, 100% and 100%. The
samples were then critical point dried in a Samdri-PVT-3B (Tousimis). The chamber was
purged a total of four times (the initial bleed + three additional
bleeds). Following mounting on stubs
with carbon dots, the specimens were sputter coated for 2.5 minutes, producing
approximately a 20 nm layer of gold palladium alloy in a Polaron sputter coater. Two
of the samples were damaged upon removal from the sputter coater. These two samples were run a second time in the sputter coater for a one-minute run.
Specimens were viewed under
standard imaging conditions at 10,000 volts on an ISI DS 130 scanning electron
microscope. Images were captured using
both a Polaroid image capture system and Semicaps
digital imaging system.
Results
Aerial and lateral views of the developing shoots were obtained.
See Figures 2 through 4 below. See the following section for a discussion
of each figure.
Discussion
Figure 3 is a low magnification aerial view of the contents of the chamber created by
the protective stipulate structure. Two
developing shoots can be observed in a shroud of trichomes. Although all of the six specimens observed
appeared to have two developing shoots, three shoots have been observed
frequently in other samples. It is quite
possible that a third shoot is present in this sample, obscured by the trichomes. The developing shoots have been interpreted
by the author, as well as others1, to consist of one dominate shoot,
a continuation of the main axis, along with branches of higher order whose
morphological derivation is under investigation. The larger, more developed shoot was interpreted as the
continuation of the main axis. This
interpretation could be further justified by determining the divergence angle
of the plant, and observing which of the shoots within the stipular structure
bears a lamina the determined number of degrees from the lamina subtending the
stipular structure, defining it as the next leaf in the helical phyllotactic
rhythm of the main shoot. The other
shoots within the stipular structure may be either axillary buds of the
subtending leaf, or a condensed branching system. The developing shoots are peculiar in that they only bear one
leaf. Axillary buds often bear numerous
leaves in a orientation similar to the plant body of the embryo. In dicotyledons, which bear two oppositely inserted
cotyledons in their embryos and early phases of development, there are often
two structures analogous to cotyledons in the axillary buds of the mature
plant, the prophylls. If after further
investigation of the axillary buds, prophylls are not found, it would be of
interest to study the early development of the plant and observe the state of
the cotyledons.
Developing
leaves have been divided into two zones: the upper leaf zone which, in many
dicotyledonous plants, gives rise to the lamina and petiole region of the leaf,
and the lower leaf zone which, in many dicotyledonous plants, develops into the
leaf base region and stipules3. The leaf base is defined as
"the widened point of insertion of the leaf
on the shoot axis." 4 Stipules are appendages of the of the leaf
base3. These concepts will prove useful in
understanding the following discussion.
Figure 4 captures a leaf
early in its development, before the stipules have begun their expansion. Two
edges of the leaf base have partially grown over the meristem of their origin, producing a cleft between them. These structures that form the cleft have been interpreted as leaf base tissue because they are confluent with the leaf base tissue near the boundary of the
upper leaf zone with the lower leaf zone. As can be seen in figure 5, "lips",
interpreted as stipule primordia,
appear to arise on this tissue. Larger
stipules at the same positions of these "lips" of more developed shoots (see
figure 6) support the interpretation of the lips as stipule primordia. As can be seen in the more developed stipules in figure 6, there is a change in growth direction between leaf base tissue, visible curving over the shoot apex, and the stipules which expand
almost perpendicularly to the inferred direction of growth of the leaf
base. It appears that leaf base tissue
grows to partially dome over the meristem of the shoot, and from this tissue,
appendages or stipules, arise. Histological
sections will add strong evidence to either support or refute these conclusions.
The "lips" in Figure 5 and
the more developed stipules in figure 6, arising on either side of the upper
leaf zone, are in the position of lateral stipules, suggesting that the mature
stipulate structure is the result of appression of two fused lateral stipules,
rather than the proliferation of a
median stipule. If the structrue were
derived from a median stipule, one would expect to see the stipule primordia
arise on the adaxial surface of the leaf near the boundary of the upper leaf
zone and the lower leaf zone.
The independent and lateral
origin of the stipules is dramatically illustrated in Figure 6. This image also suggests that the leaf base
has not only grown to partially cover the meristem that generated it, but that
it has grown to encircle the meristem. The leaf base edges have become
confluent to form a tube by the processes of "meristem incorporation and
meristem fusion."2More evidence is needed to confirm this observation. A dome, which is here
interpreted as the apical meristem, is visible in the cleft created by the leaf
base tissue.
Figure 7 captures yet a
later stage in development in which the stipules have increased markedly in
size and appear to be in the process of becoming appressed. It is unknown if the tissues of the stipules intercalate to form pseudoparenchyma, but it is impossible to come to a
conclusion about this aspect of stipule development from the electron
micrographs obtained, thus the term "appressed" is used instead of "fused".
In this figure, the stipules have taken on
the gross form of the protective keele-shaped structure, differing from the
mature form mostly in size. A zone of
tissue damage is evident to the right of the stipular structure because the
lamina and petiole of this leaf were broken off after sputter coating as
alluded to above.
The organ enclosing the
apical meristem and its developing derivatives in Exbucklandia populnea has its
origin as two symmetric lateral stipules on a leaf base that grows to encircle
its generitive meristem. The stipules
originate as what have been termed "lips" on the leaf base tissue, elongate
independently and symmetrically, and finally become appressed to form the
mature structure.
There
was a noticeable difference in the preservation of trichome structure. Compare the trichomes in figure 7 in which
they appear flacid and distorted and the trichomes in figure 5, in which they
appear more robust. The difference
could be an artifact of tissue preparation, the use or non-use of osmium
tetroxide, or just differences that were in the trichomes before they were
collected. Unfortunately this cannot be
determined from data collected at the apices treated with osmium tetroxide were
mixed with the untreated shoot apices before sputtercoating. Thus, it was impossible to asses the benefit
of using the osmium.
References
-
Das, Gitasree., A.K. Das. 1984. Ordering of shoots with special reference to Exbucklandia populnea. Current Science. May 5, 1984: 498-499.
- Hagemann in Kaplan, D. R. The Principles of Plant Morphology, Volume III. Berkeley: Odin Readers, 1999: Chapter 15.
- Kaplan, D. R. The Principles of Plant Morphology, Volume III. Berkeley:
Odin Readers, 1999: Chapter 15.
- Troll in Kaplan, D. R. The Principles of Plant Morphology, Volume III.
Berkeley: Odin Readers, 1999: Chapter 15.
Figures
Figure 1.

A Leaf of Exbucklandia and a portion of the "stem" on which it is born. The artificial boundary between the leaf and "stem" in this plant is a the insertion point of the stipular structure. Ax, axis; L, lamina; P, petiole; St, stipules.
Figure 2.

A close-up of the stipular structure. Ax, Axis; P, petiole; St. stipules
Figure 3.

Aerial view of the main axis apex with the stipular structure removed. Note two developing shoots in a mass of trichomes. The point of insertion of the upper leaf associated with the removed stipules is out of the frame to the top. Note the stipular scars.
Figure 4.

Aerial view of a shoot in an early stage of development. Note the cleft between the two zones of leaf base expansion. ULZ, upper leaf zone.
Figure 5.

Aerial view of a shoot apex bearing stipule primordia which are evident as
"lips" on the leaf base tissue. ULZ, upper leaf zone.
Figure 6.

Lateral view of a developing shoot. The stipules are seen expanding independently. The apical meristem is visible in the cleft. Some of the stipular structure that enclosed this shoot is evident in cellular sheet in the background. AM, apical meristem; St, stipules; ULZ, upper leaf zone.
Figure 7.
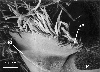
Oblique view showing stipules becoming appressed at margin to form the stipular structure. Note the light colored stipule insertion point. P, petiole scar (upper leaf zone broke during processing); SI, stipule insertion point, SA, stipule appression at the margin.






