One of the major questions in development is how cell fate is determined and how patterns are established. The maize leaf provides an elegant and tractable model for examining cell fate determination and pattern formation. Its development is well characterized, and its use as a genetic system is well established (Sharman, 1942; Poethig, 1984; Sylvester et al., 1990). A typical maize leaf is divided into three regions: the proximal sheath, the distal blade, and the ligular region, which forms at the blade/sheath boundary. The ligular region is composed of the ligule and auricle. The ligule is an epidermal fringe of tissue, which extends from the adaxial surface of the leaf. The wedged-shaped auricles act as hinges and allow the blade to project at an angle from the maize axis.
Leaves form as lateral outgrowths flanking the shoot apical meristem (Steeves and Sussex, 1989). Leaf development can be described in three phases: 1) initialization of leaf founder cells in the meristem, 2) founder cell division that gives rise to leaf primordia, and 3) longitudinal differentiation that occurs basipetally as well as lateral differentiation from the midrib to the margin (Harper and Freeling, 1996b). The first visible sign of ligule region development is an increase in anticlinal cell divisions on the adaxial epidermis of young leaf primoridia (Sylvester et al., 1990). The anticlinal cell divisions give rise to columnar cells that divide periclinally to give rise to the ligule ridge (Becraft et al., 1990). The anticlinal divisions occur synchronistically within the region that will give rise to the ligule and auricle, while the periclinal divisions occur progressively from the midvein to the leaf margin (Sylvester et al., 1990).
Although the ligule has been well characterized via SEM analysis comparing wildtype and liguless1 (lg1) leaf development (Becraft et al., 1990, Sylvester et al., 1990), auricle development has been largely ignored. However, auricle development has been described in conjunction with ligule development (Becraft et al., 1990). The auricle is first visible as a thin line of cells that separate the blade and sheath. This line of cells is only visible after initiation of the ligule. The auricle mesophyll cells enlarge concomitantly with ligule outgrowth. Then, blade and sheath expand while the auricle cells divide. After the leaf emerges, the auricle cells expand allowing the leaf blade to bend out horizontally from the main axis.
We have identified a novel mutant that we call extended auricle (eta). eta is a recessive mutant that was found in an EMS screen. Mutant eta individuals have a wavy overgrowth of auricle tissue and the auricle/blade boundary is diffuse. In addition, aberrations can be seen in the ligule in successively older leaves. The ligule fringe forms normally over the midvein, but is interrupted as the ligule extends marginally. There are also overgrowths of tissue at the ligule region that appear to be non-ligular (i.e. non-epidermal). Also, abnormal tendril-like structures have been noted on the adaxial surface of lg1/eta leaves.
Preliminary evidence from super glue impressions indicates that there is an increase in the number of auricle cells in the mutant relative to wildtype. This suggests that there is either a transformation in cell fates at the blade/sheath boundary or an over-proliferation of auricle tissue. Auricle tissue is distinct from blade, sheath, and ligule based on cell shape, size and autofluorescence this allows determination of cell types in the mutant tissue. The aims of this project are to use SEM to observe differences between the cell type, size, and shape of wild type and eta leaves and to characterize the morphological abnormalities in eta/lg1 leaves.
Families segregating the eta mutation in both the Mo17 and B73 backgrounds were used in this study. These plants were grown in the greenhouse and harvested at seven weeks. In addition, double mutant families between lg1 and eta were planted for observation. These families were grown in the field and leaf samples were collected at eight weeks.
Leaf tissue samples were fixed in 2% glutaraldehyde in 0.1M sodium cacodylate buffer, pH 7.2 for 1 hour under vacuum. They were then rinsed in sodium cacodylate buffer three times over the course of 45 minutes. This was followed by post-fixation in 1% Osmium tetroxide in sodium cacodylate buffer for one hour. The samples were then rinsed in sodium cacodylate buffer three times over the course of 15 minutes. The samples were taken through an ethanol dehydration series (35%, 50%, 70%, 80%, 95%, 100%, 100%); ten minutes at each step. The samples were then critical point dried, mounted onto stubs using carbon dots, and sputter coated with gold-palladium (Bozzola and Russell, 1999). An ISI DS-130 scanning electron microscope was used to visualize the samples.
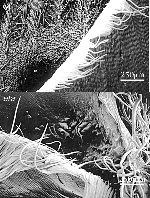
The first aim in this study was to observe differences in cell type, size and shape between wildtype and eta leaves at the ligule/ auricle boundary in two different backgrounds, Mo17 and B73. The eta phenotype is considerably more severe in the Mo17 than the B73 background. The leaf organization in wildtype Mo17 can be seen in Figure 1A. Along proximal-distal axis, sheath tissue, ligule tissue, auricle tissue and blade tissue is clearly visible. In contrast Figure 1B shows the eta mutant at the blade/sheath boundary. Sheath cells and organization are normal but the ligule fringe of epidermal cells are relatively unfused. In addition, the boundary between the auricle and blade is unclear. The same is true for the organization of eta mutant leaves relative to their wildtype siblings in B73 (see Figure 2).
In both the Mo17 and B73 backgrounds there are abnormal , bumpy cells in the auricle region toward the margin (see Figure 1B and Figure 3). There is also an increase in macrohairs along the margin of eta individuals in both Mo17 and B73 (see Figure 1B and Figure 3). We also used SEM analysis to better observe abnormal structures on the abaxial blade surface of eta/ lg1 double mutants. These structures are visible with the naked eye and are visible on both the abaxial and adaxial leaf surfaces (See Figure 4).
The SEM analysis in this study was useful in several respects, but also generated as many questions as it answered. First of all, it seems there is the same organizational defect in both the Mo17 and B73 backgrounds, with the auricle/ blade boundary very diffuse. Both an increase in macrohairs along the leaf margin and abnormal auricle cells can be seen in Mo17 and B73; these findings were unexpected, but insightful. Perhaps these abnormal auricle cells give rise to the large outgrowths on the abaxial surface of lg1/eta double mutant leaves when the ligule is not present. Future studies will focus on developing auricles and ligules in eta vs. wildtype siblings to establish the developmental time point in which the eta phenotype can first be seen.