The fixation and dehydration of plant tissues for electron microscopy pose distinct challenges when compared to the preparation of animal or microbial samples (Jakstys, 1999). Among the characteristics that complicate reagent penetration into the samples are the thick cuticular waxes on much of the plant epidermis, internal air pockets (e.g within leaves), and trichomes/tissue ridges that tend to trap air and prevent the tissue sample from sinking into the liquid. Among the techniques employed to overcome such difficulties are longer fixation and dehydration times (Bozzola and Russell, 1999) and the use of intermittent vacuum treatment (Jakstys, 1999).
Among the artifacts introduced into plant scanning electron microscope specimens during conventional preparation are distortion of the epidermis and collapse of fine structures; to overcome this problem some plant biologists make impressions of the tissue and mold resin casts for viewing (Williams and Sylvester, 1994). Use of the microwave oven to expedite tissue preparation and minimize artifacts is an attractive alternative to conventional specimen handling for the viewing of original samples. However, since sample preparation can vary depending on the individual's skill and material type, it was necessary to compare the two techniques side-by-side on similar samples in order to assess their relative properties with respect to fine structural detail.
The adaxial maize leaf surface is divided into three regions: the distal blade, the proximal sheath, and the ligular region. The ligule is a fine ridge of epidermal cells that separates the blade and sheath tissue types. Among the effects of the morphological mutant extended auricle1 (eta) is the disruption of the continuity of the ligule (Osmont and Freeling, 2001). Thus, the analysis of the eta mutant requires a high degree of detail and a minimum of fine structure distortion, and is well-suited to a comparison of tissue preparation techniques.
For this study, wild-type leaves and mutant eta leaves in the Mo17 background were removed from 6-week old plants and various developmental stages were identified by taking leaves progressively closer to the shoot apex. The ligular region was cut away from the rest of the leaf and immediately placed in cold fixative buffer.
Tissues were fixed in 2% glutaraldehyde in 0.1 M Na-Cacodylate buffer, pH 7.2 and subjected to a vacuum for 1-4 minutes every 15 minutes for 2 hours on ice. Prior to vacuum treatment, floating samples were poked under the buffer surface with pointed metal pokers. Rinsing took place in 0.1 M Na-Cacodylate buffer, pH 7.2, for 45 minutes, with buffer changes at 15 and 30 minutes. Further fixation in 1% Osmium Tetroxide in Na-Cacodylate buffer, under intermittent vacuum and poking, took place for 1.5 hours. Samples were then rinsed again in the Na-Cacodylate buffer. Samples were dehydrated through an ethanol series in buffer: 35% - 50% - 70% - 80% - 95% - 100% - 100% for 15 minutes each.
Tissues were fixed in the same glutaraldehyde solution at 37C in the microwave oven twice for 40 seconds each; in between microwaving, floating samples were poked under the surface. Samples were rinsed twice for 2.5 minutes in Na-Cacodylate buffer at room temperature, and fixed in 1% Osmium Tetroxide for 20 seconds at 37C in the microwave and quickly cooled back to room temperature. The second buffer rinse was 30 seconds, followed by a series of ethanol dehydration steps in the microwave at 45C: 50% - 70% - 90% - 100% for twice 40 seconds at each step.
Immediately upon the completion of dehydration, all samples were dried in a critical-point dryer. Later, the samples were mounted on stubs and coated to a thickness of 22 nm with Au-Pd in a sputter coater. Prepared samples were stored in a dessicator prior to viewing. In total, seven tissue samples (4 conventionally-prepared, 3 microwave-prepared) were mounted and examined.
Samples were either viewed on the ISI DS-130 scanning electron microscope or the Hitachi S-5000 scanning electron microscope.
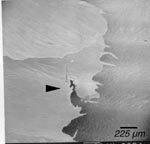
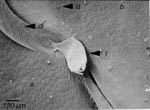
Due to the considerable amount of poking the conventionally-prepared tissue experienced (see Materials and Methods), it was expected that such tissue would exhibit a greater extent of damage than the microwave-fixed samples. Indeed, the conventionally-prepared specimens overall showed a qualitatively larger amount of tearing (Fig. 1) and smashing (Fig. 2). However, similar structures were also seen, though less severe, in microwave-prepared specimens (Fig. 3). This observation may be due to the fact that microwave-prepared samples did experience some poking, although much less than the conventional samples or the damage seen in both sets may be due to handling or effects of other steps in the preparation protocol, including mounting. It was observed that mounting a naturally curled tissue onto a flat stub surface could result in tissue breakage (Fig. 4).
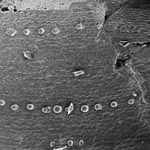
Another area in which microwave fixation and dehydration might improve upon conventional preparation is in the preservation of fine detail, such as in the single-celled hairs protruding from the leaf ligule. Typical SEM preparation techniques tend to damage such hairs, which end up appearing bent and deflated (Osmont, personal communication). Although both bent and properly protruding hairs were observed (Fig. 5), it is not possible with so few samples to assign them to a particular treatment.
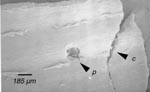
To characterize the ability of the SEM to reveal fine structure in developing wild-type and eta mutant ligules, regions of mutant ligule disruption and wild-type ligule were photographed and compared (Fig. 6). Despite technical problems such as damage to fine single-celled structure and the accumulation of small debris on the leaf surface, the micrographs provide adequate information for characterizing the mutant's gross effects on ligule morphology. The mutant ligules are discontinuous compared with the wild-type, but the small sections of ligule appear relatively normal. The mutant ligule phenotype warrants further study.
Despite microwave fixation's obvious advantages in speed for the preparation of plant material, it was not substantially better than conventional preparation. Although the latter protocol resulted in apparently more and qualitatively more destructive damage, it is also possible that other aspects of tissue handling resulted in damage, as both sets experienced some degree of damage. It is potentially possible to modify conventional or microwave preparation (by adding tannic acid and uranyl acetate processing steps, as in Hanschke and Schauer, 1996) in some types of tissue. Since the ligular region is inherently ridged, thus trapping numerous air bubbles and floating the specimen, it will always prove challenging to prepare such tissue in liquid solution. For the preservation of gross anatomy, alternative techniques, such as dental-impression molding of tissue (Williams and Sylvester, 1994) or the use of an environmental scanning electron microscope may be preferable.